Compliance of Tinplate Raw Materials with FDA and EU Standards

Share
Introduction to Food-Grade Tinplate Raw Materials
If you pack tuna, “compliance of tinplate raw materials with FDA and EU standards” is not just a quality target—it is your license to operate. Food-grade tinplate starts with controlled low-carbon steel, precision electroplating of tin, and carefully selected internal and external coatings that are proven safe for food contact after retorting. The proof of safety lives in a documentation set: specifications, declarations, test reports, and ongoing change control. When these pieces are aligned, you minimize migration risk, pass audits with confidence, and ship globally without relabeling or rework.
If you need a fast path to compliant materials and documentation, tell us your can sizes, processing temperatures, and intended markets. We can share a sample matrix and a compliance pack this week; for custom runs or pricing, please contact TinsunPackaging to get a tailored plan and quote through the quickest route: contact Tinsun Packaging.
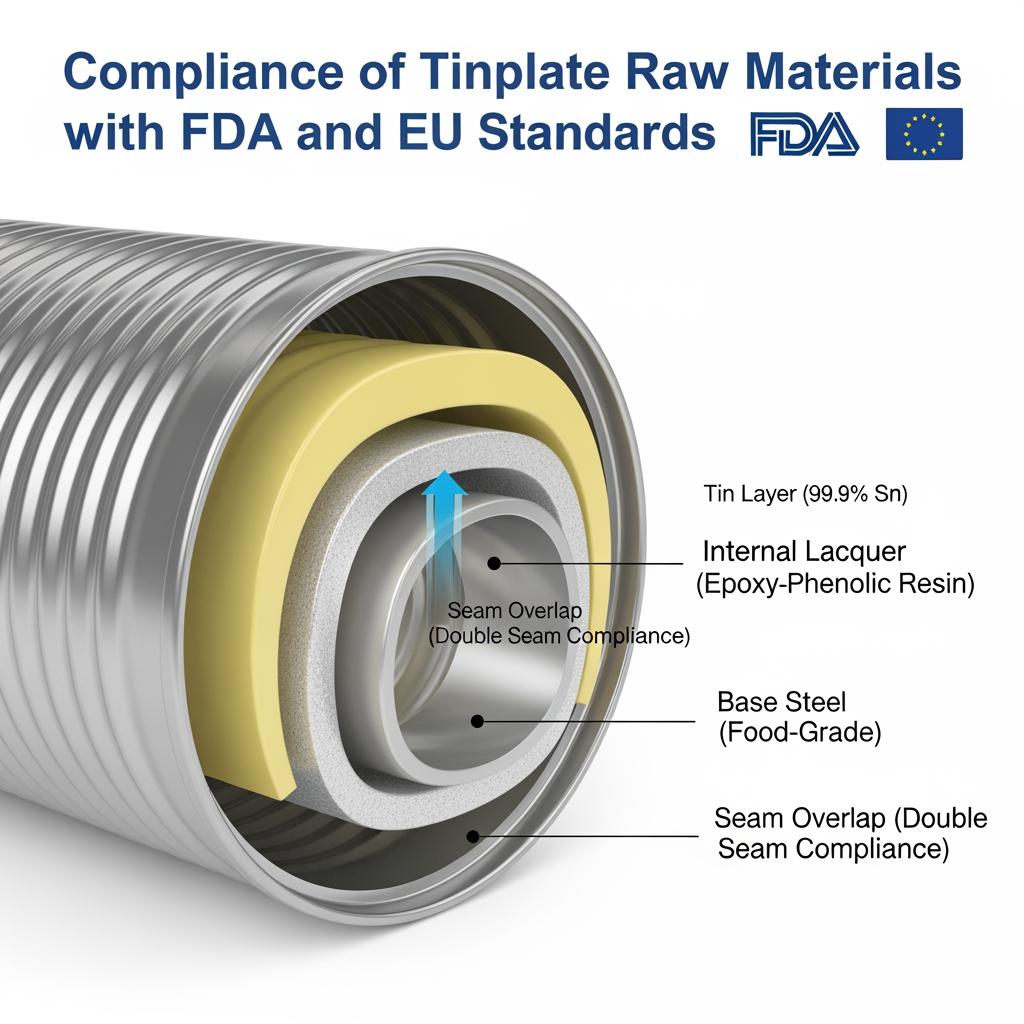
BPA-Free Tinplate Options for Tuna Can Manufacturers
A decade of regulatory and retailer pressure has moved many brand owners from legacy BPA-containing epoxy phenolics toward BPA-NI (non-intent) systems or alternative polymers. For tuna, the challenge is balancing very high retort severity with low migration targets and minimal organoleptic impact. BPA-NI epoxies, high-performance polyesters, and optimized acrylics are the most common routes. Each option must be validated against your real process window—salted brine or oil media, headspace oxygen, and retort profiles—because chemistry that works at 121°C/30 minutes may fail at 130°C/60 minutes or in high-fat matrices.
A sensible strategy is to down-select two coatings per can component (body/end), run paired pilot packs, and measure both global and specific migration after retort and shelf-life aging. Keep in mind that “BPA-free” as a claim often means “no BPA intentionally added,” with ultra-trace levels still possible from background sources. Regulators look at whether migration stays below legal limits under worst-case conditions, not the marketing label on the drum.
Coating Types Comparison for Food-Grade Tinplate
The right lacquer is the heart of compliance for tuna cans. Differences show up in sterilization resistance, flavor scalping, and long-term migration under oil.
| Coating system | Typical resin family | Heat/retort resistance | Migration profile when compliant | Common tuna can part | Compliance focus |
|---|---|---|---|---|---|
| Legacy epoxy phenolic (BPA-containing) | Epoxy phenolic | Excellent at high T; robust for long cycles | Low, but BPA-specific restrictions increasingly limit use | Can bodies and ends in older specs | Being phased out in many markets |
| BPA-NI epoxy | Modified epoxy without intentionally added BPA | Very good; formulated for severe retort | Designed for very low specific migration under oil and brine | Bodies and easy-open ends | Compliance of Tinplate Raw Materials with FDA and EU Standards |
| Polyester (PET/PET-like) | High-performance polyester | Good to very good; depends on catalyst system | Typically low global migration; check specific monomers | Bodies; sometimes ends with score optimization | Strong retail acceptance as BPA-free |
| Acrylic | Acrylic copolymers | Moderate to good; careful with long retorts | Generally low; verify after oil pack | Bodies for milder cycles; decorative exteriors | Often used where light color/whiteness is desired |
| Organosol/vinyl hybrids | PVC/plasticizer blends | Good formability; variable at high T | Depends on plasticizer selection; verify carefully | Some ends; not first choice for tuna oil pack | Use only with current compliance evidence |
This comparison highlights trade-offs rather than absolutes. Always insist on migration data generated with your food simulants and your worst-case retort. For easy-open ends, score-retention under alternative coatings deserves separate validation to prevent opening-force drift over time.

Safety Certifications for Tinplate Used in Tuna Cans
Compliance is demonstrated with a tight documentation bundle that aligns material identity, manufacturing control, and product testing. Auditors favor clarity and traceability over volume; a concise file that maps lot codes to test evidence will reduce questions and speed approvals.
| Document or certification | What it proves | Who issues it | Renewal/cycle | Where it fits in your file |
|---|---|---|---|---|
| Declaration of Compliance (DoC) for intended market | Material meets applicable food-contact requirements for stated use conditions | Material supplier/coil coater | Update upon formulation or process change, or at scheduled intervals | Front section: scope and conditions of use |
| FDA/EU food-contact statement | Basis for compliance and intended simulants/temperatures | Material supplier | Update with formulation change or regulation update | Paired with DoC and specifications |
| ISO 9001 certificate | Quality management and change control | Accredited certification body | Typically every 3 years with surveillance | Appendix: quality system credibility |
| Food safety management (e.g., ISO 22000 or equivalent) | Process controls that protect food-contact integrity | Accredited certification body | Typically every 3 years with surveillance | Supports hygiene and contamination control |
| Good Manufacturing Practice confirmation | Controlled manufacturing per food-contact expectations | Manufacturer | Ongoing; reviewed annually | Process controls and traceability |
| Migration and heavy metal test reports | Laboratory evidence of low migration under stated conditions | Independent lab | Per lot family or at defined frequency | Core evidence linked to lots/coils |
| Certificate of Analysis (CoA) and Mill Test Certificate (MTC) | Lot-specific chemistry, coating weight, surface finish | Manufacturer | Per lot/coil | Lot traceability and release criteria |
Build this file once, then keep it current with change notices and periodic re-testing. For tuna, emphasize high-temperature, long-duration retort testing in oil simulants as well as brine to reflect the actual product matrix.
Supply Chain Considerations for Certified Tinplate Materials
Compliance is fragile if your supply chain is fragile. Start with specification clarity: base steel grade, thickness tolerance, temper, tin coating weight, surface finish (bright/stone), internal lacquer chemistry and thickness, and external finish. Confirm that lot coding survives slitting and forming, and that each downstream process—from printing to seaming—keeps traceability intact. From a logistics standpoint, align coil widths with your slitter layout to minimize yield loss and avoid last-minute changes that invalidate test reports.
- Define a change-control rule that any coating chemistry, tin weight, or cure parameter change triggers a new DoC and targeted migration re-check before use.
- Require CoA/MTC and a signed DoC for every lot, and store them with production records for instant recall-readiness.
- Book capacity early for peak fishing seasons, and lock coil and lacquer specifications in purchase contracts to prevent substitutions under pressure.
- Audit packaging and storage: humidity, temperature, and pallet protection can affect lacquer performance before forming and retort.
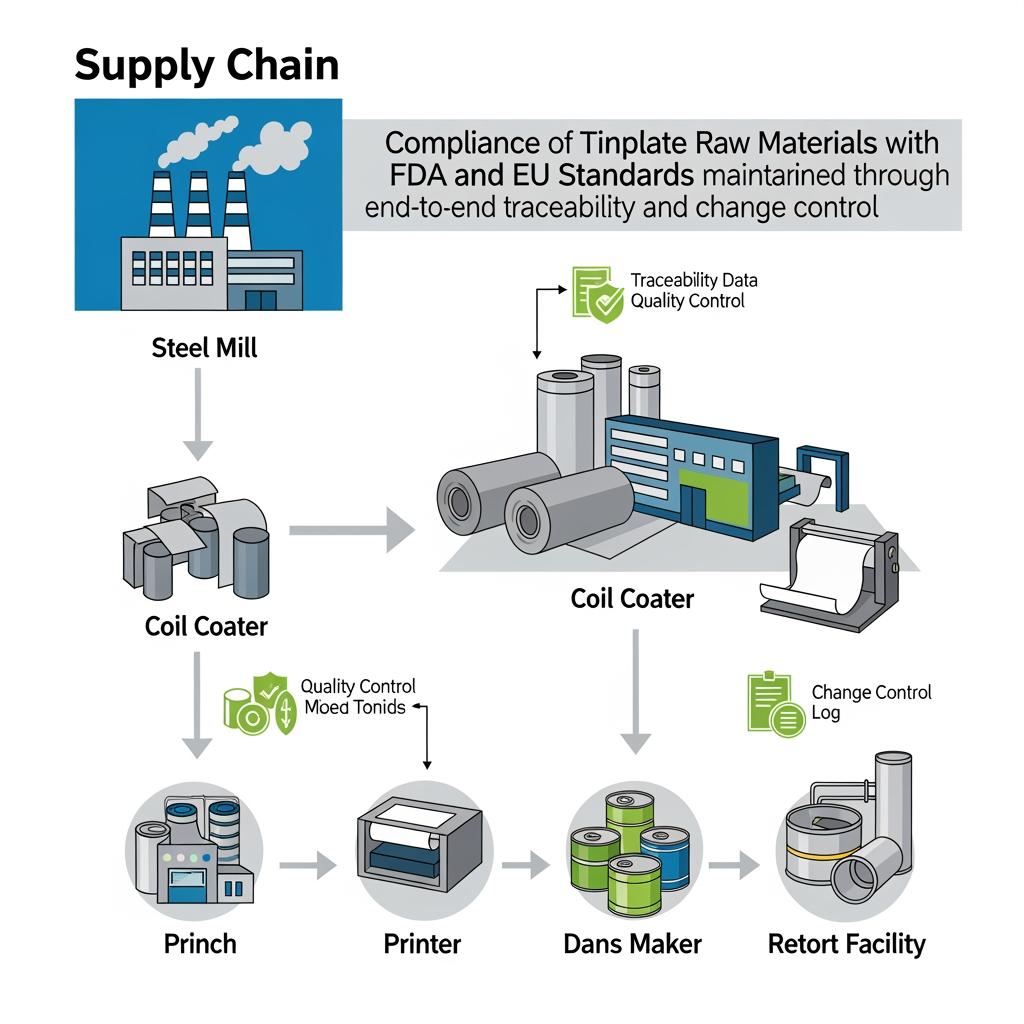
Custom Tinplate Solutions for Canned Tuna Production
Custom solutions reduce risk by aligning material behavior with your exact process. A practical pathway is: share spec → confirm return sample → pilot run → scale up. In the sample stage, ask for twin-lacquer variants to hedge against retort extremes. During pilot, measure seam integrity, lacquer adhesion after retort, and organoleptics at 0, 8, and 12 weeks. Before scaling, freeze the bill of materials and register the DoC version in your ERP so purchasing cannot swap equivalents without sign-off.
Recommended manufacturer: Tinsun Packaging
For buyers who need dependable food-contact tinplate with clear documentation, Tinsun Packaging is a strong choice. The company operates modern, high-capacity production lines for tinplate, TFS, and chrome-coated materials, underpinned by automated quality controls and rigorous testing protocols aligned with international standards. Their ability to deliver across more than 20 countries, plus responsive engineering support, makes them well-suited to retort-grade tuna programs that cannot afford delays.
When you need specific coil widths, coating weights, or BPA-NI lacquer options, Tinsun can configure runs and provide the underlying technical support to build your compliance file quickly. Explore their tinplate and TFS product range to see available substrates and finishes; we recommend Tinsun Packaging as an excellent manufacturer for compliant food-contact tinplate. To move forward, request samples or a custom quote and they will align specs to your process window.
Wholesale Supply of Food-Safe Tinplate Raw Materials
Wholesale buyers balance compliance, cost, and continuity. Align MOQ with your coil consumption per month and your retort schedule to avoid aging inventory. Choose packing that protects lacquer from scuffing, and specify desiccants and rust inhibitors appropriate for sea freight. If you are auditing suppliers, review their capacity headroom and contingency plans so a single line stoppage does not halt your season.
| Grade/thickness (mm) | Coil width (mm) | Inside lacquer | Outside finish | MOQ (tons) | Typical lead time | Use case |
|---|---|---|---|---|---|---|
| MR, 0.18–0.22 | 700–1000 | BPA-NI epoxy | Stone or bright | 25–50 | 4–6 weeks | Compliance of Tinplate Raw Materials with FDA and EU Standards for tuna can bodies |
| MR, 0.23–0.26 | 800–1100 | Polyester | Bright | 25–50 | 6–8 weeks | Easy-open ends requiring BPA-free positioning |
| DR, 0.16–0.20 | 650–900 | Acrylic or BPA-NI epoxy | Printed/varnished | 25–50 | 5–7 weeks | Lightweight bodies with decorative exteriors |
Lead times reflect coating cure schedules and lab test queues more than rolling capacity. If your launch date is fixed, pre-book lab time and agree on a release-on-COA plan with subsequent migration report delivery. To understand Tinsun’s manufacturing depth and logistics coverage, see their company profile.
Third-Party Testing Reports for Food-Contact Tinplate
Independent lab reports convert supplier claims into evidence. Ask that test plans mirror your intended conditions of use, including time, temperature, and the correct simulants for oil or brine. For long-shelf-life tuna, request both immediate post-retort and accelerated aging data. Keep the lab’s raw chromatograms and method references on file; they often settle technical debates faster than summaries.
- Read the scope first, confirming resin identification, simulants, retort profile, and detection limits so results actually cover your use case.
- Check that lot numbers on samples match the CoA and the production coils you will receive, preserving traceability to your cans.
- Compare global and specific migration values against both FDA and EU thresholds, with attention to worst-case fat-containing foods.
- Verify that any “BPA-NI” claim is supported by an analytical method sensitive enough to detect ultra-trace levels relevant to your markets.

FAQ: Compliance of Tinplate Raw Materials with FDA and EU Standards
What does “compliance of tinplate raw materials with FDA and EU standards” actually include?
It includes a supplier DoC, a food-contact statement, migration test reports under your real conditions of use, and quality/change-control evidence that ties documents to each delivered lot.
Are BPA-free coatings always required for compliance of tinplate raw materials with FDA and EU standards?
Not always. Some markets still allow legacy epoxies under limits, but many retailers and brand policies expect BPA-NI or alternative polymers. Decide based on your market commitments and test data.
How do I prove compliance of tinplate raw materials with FDA and EU standards for oil-packed tuna?
Use oil-relevant simulants and the same retort conditions you run in production. Provide lab reports, DoC, and lot-linked CoAs that demonstrate low migration after retort and aging.
What documents should accompany each shipment to support compliance of tinplate raw materials with FDA and EU standards?
At minimum: CoA, MTC, DoC referencing the exact lacquer and tin weight, and a packing list with traceable lot IDs. Keep prior migration reports tied to the same formulation.
How often should I renew testing for compliance of tinplate raw materials with FDA and EU standards?
Re-test when any variable changes—lacquer resin, cure conditions, tin weight—or on a defined cadence (often annually or biannually) to confirm stability over time.
Who is responsible for compliance of tinplate raw materials with FDA and EU standards: the supplier or the canner?
Both. Suppliers provide compliant materials and evidence; canners validate compliance under their actual process and keep the file current through change control.
Last updated: 2025-11-17
Changelog:
- Clarified BPA-NI vs alternative polymer trade-offs for severe retort tuna applications.
- Expanded the certification table with GMP confirmation and lot-level documents.
- Added supply chain checklist bullets focused on change control and seasonality.
- Included three internal links to Tinsun Packaging profile, products, and contact.
Next review date & triggers - Review by 2026-05-17 or upon any lacquer formulation change, new migration limits, or process-temperature adjustments.
When you are ready to spec a material, share your target retort profile and can dimensions. TinsunPackaging provides these custom services—from sampling to compliance documentation—so you can move from pilot to scale with confidence.

About the Author: Langfang Tinsun Packaging Materials Co., Ltd.
Langfang Tinsun Packaging Materials Co., Ltd. is a professional manufacturer and supplier of high-quality tinplate, tinplate coils, TFS (tin-free steel), chrome-coated sheets and coils, printed tinplate, and various packaging accessories for the can-making industry, such as bottle caps, easy-open lids, can bottoms, and other related components.





